Articles from CardioFocus, Inc.

CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, today announced a series of major milestones advancing its pulsed field ablation (PFA) program. The company has initiated its CE Mark pivotal clinical trial for the QuickShot™ Nav large-area focal PFA Catheter and the next-generation CardioWave™ PFA System. The first patients were enrolled at KBC Split in Croatia under the leadership of Dr. Ante Anić and included the first-ever magnetically tracked QuickShot cases.
By CardioFocus, Inc. · Via Business Wire · November 11, 2025

CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, is excited to announce its featured presence at Heart Rhythm 2025 in San Diego, where the company will showcase the latest advancements and clinical progress in pulsed field ablation (PFA) technology for the treatment of atrial fibrillation.
By CardioFocus, Inc. · Via Business Wire · April 22, 2025

CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, announced the continuation of the Quick AF study, which evaluates the investigational QuickShot™ Nav large-area focal pulsed field ablation (PFA) catheter for the treatment of patients with persistent atrial fibrillation. The cases were performed by Dr. Ante Anić at KBC Split in Split, Croatia.
By CardioFocus, Inc. · Via Business Wire · April 16, 2025
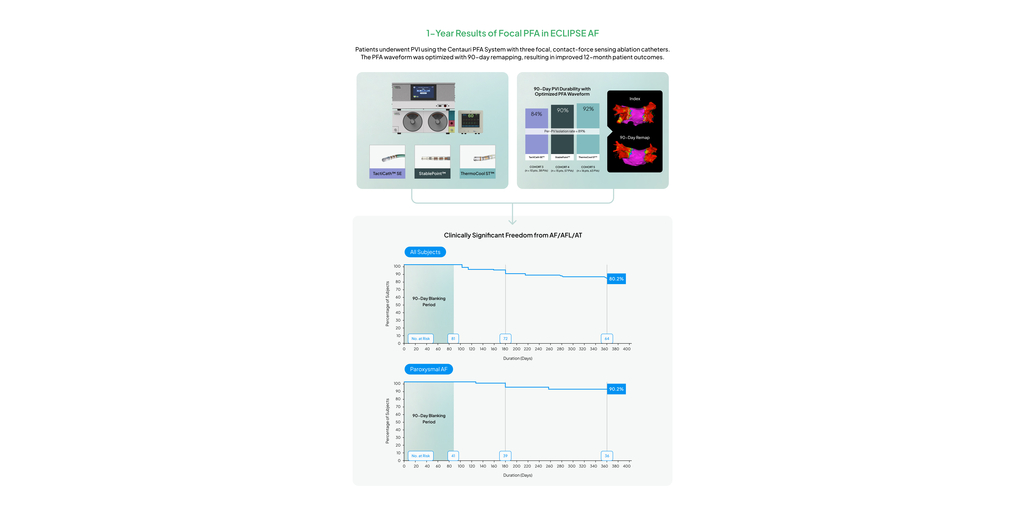
CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, today announced the publication of the 12-month results from the ECLIPSE AF trial in Circulation: Arrhythmia & Electrophysiology, demonstrating an overall 90.2% 12-month freedom from clinically significant atrial arrhythmia among paroxysmal atrial fibrillation (AF) patients undergoing de novo pulmonary vein isolation (PVI) using three commercial contact-force sensing focal catheters with the Centauri™ System’s optimized WAVE1™ pulsed field ablation (PFA) waveform. The system, which has since received CE mark and is commercially available within the EU and UK where over 6000 patients have been successfully treated, was used with the Abbott TactiCath™ Sensor Enabled, Boston Scientific INTELLANAV STABLEPOINT™, and Johnson & Johnson THERMOCOOL SMARTTOUCH™ ablation catheters. Paroxysmal and persistent AF patients were treated in the study and demonstrated an overall 80.2% 12-month freedom from clinically significant atrial arrhythmia. The waveform was optimized with invasive 90-day remapping and demonstrated an overall chronic PVI durability rate of 89%, per pulmonary vein, in patients treated within the optimized PFA cohorts.
By CardioFocus, Inc. · Via Business Wire · January 14, 2025
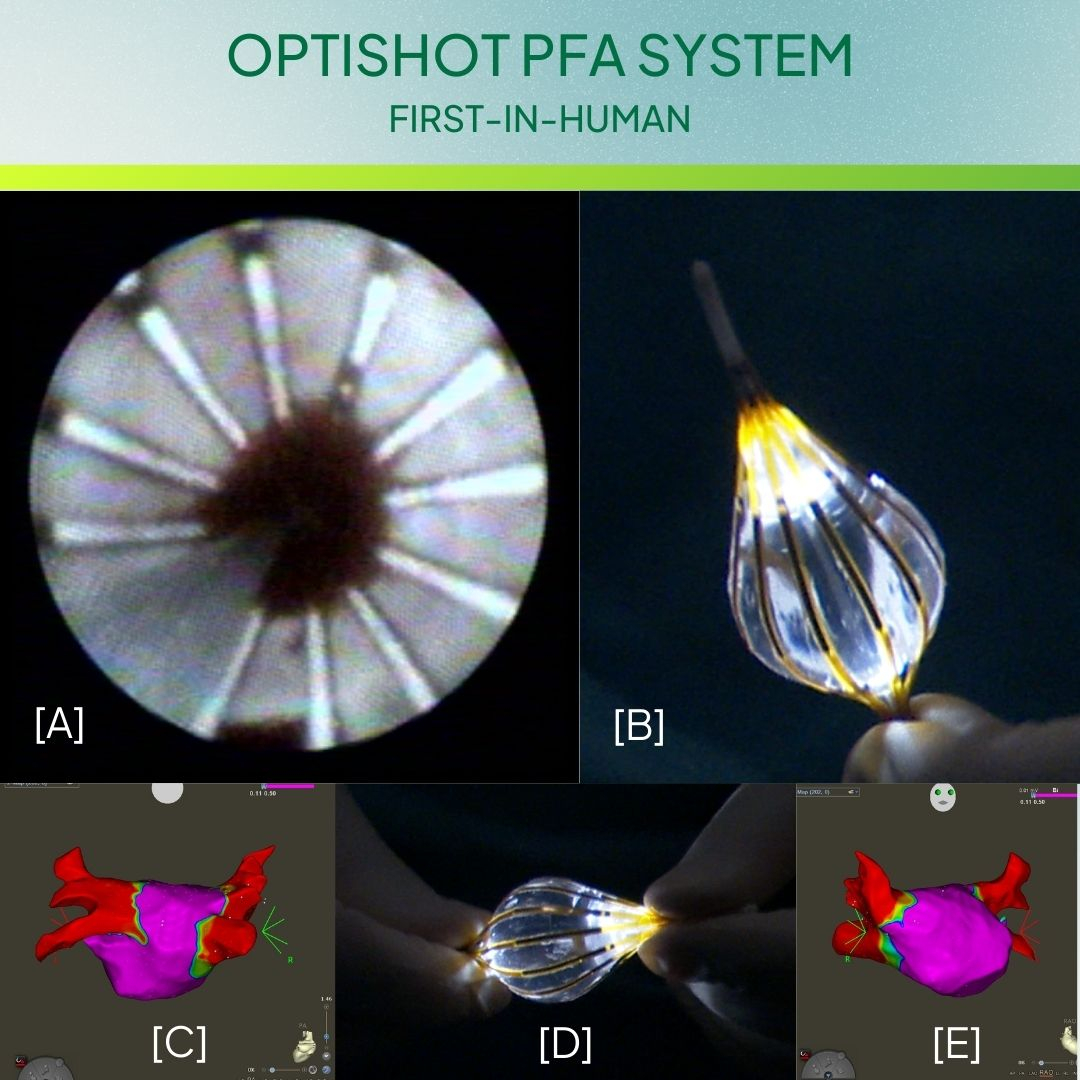
CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, today announced the first series of patients treated with the investigational OptiShot™ Pulsed Field Ablation (PFA) System for the treatment of paroxysmal atrial fibrillation as part of the VISION AF clinical trial.
By CardioFocus, Inc. · Via Business Wire · December 17, 2024
